By John Vandermosten, CFA
READ THE FULL MDAI RESEARCH REPORT
Spectral AI, Inc. (NASDAQ:MDAI) reported topline data from its burn validation study in a March 17th press release confirming DeepView’s superior detection sensitivity relative to burn physicians. The announcement reminded stakeholders of the device’s background and development along with a description of the burn study’s trial design. Data regarding DeepView’s sensitivity, specificity and Dice Score were provided. Submission of the De Novo application to the FDA is expected in mid-2025. Spectral communicated other important information recently including the reiteration of access to additional financing, three device installations in Australia, progress in the intended spin-off of Spectral IP and the announcement of its 2024 financial results on March 27th.
Burn Validation Study Results
On February 7th, Spectral announced that it had completed the data analysis of the burn validation study presaging the release of topline data on March 17th. Results comparing the sensitivity, Dice score and specificity were provided along with anticipated timing for clearance and commercialization.
Burn Trial Background
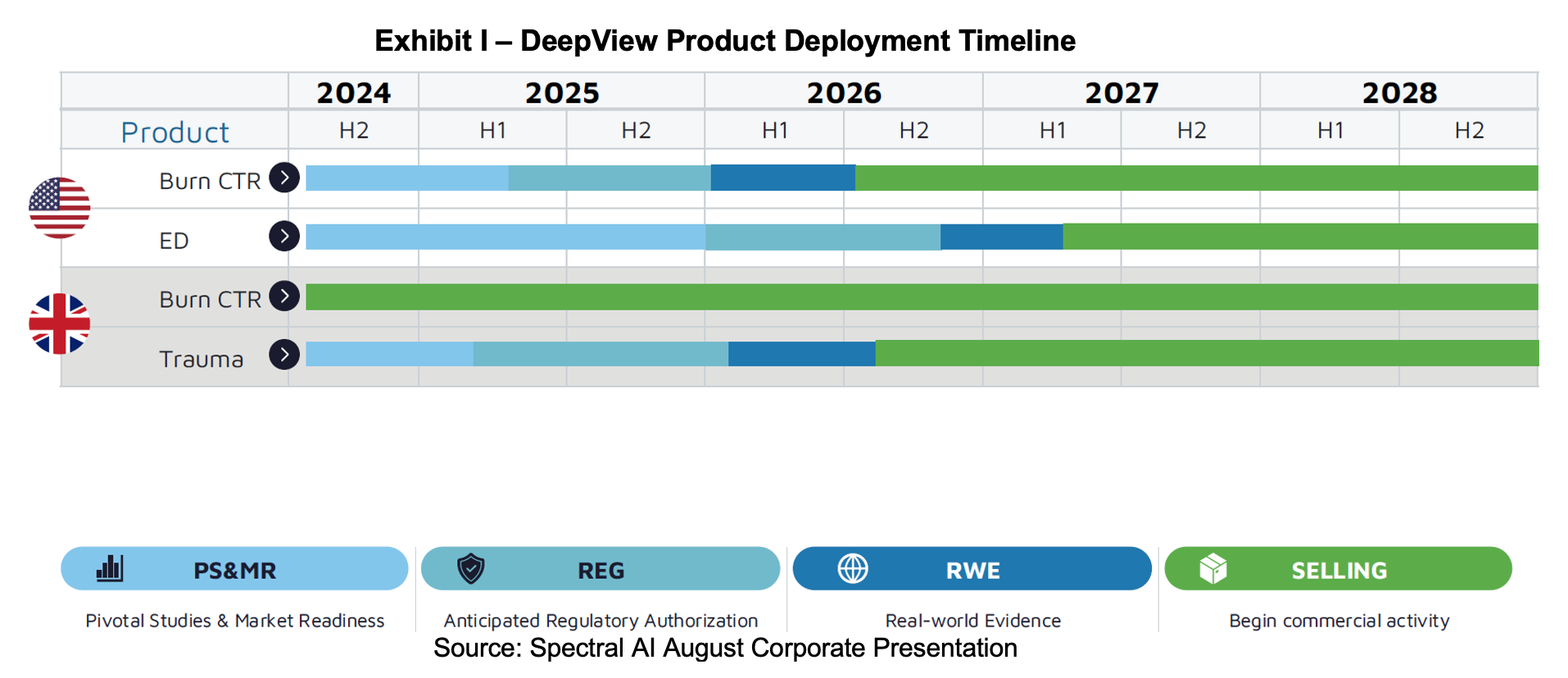
On January 11th, 2024, Spectral announced that it began enrollment of its pivotal study to validate DeepView for burn injuries. The study enrolled and analyzed data from 164 patients, consisting of 115 adults and 49 children in burn centers and emergency departments throughout the US. The study was filed under clinicaltrials.gov under the designator NCT06131203 and is titled Burn Pivotal Study. Its purpose is to validate the burn healing assessment algorithm for the DeepView device. 16 sites enrolled patients.
Analysis of study data generated results related to the sensitivity, specificity and Dice score of the DeepView system at both a pixel level and full image level compared with the clinical judgment of burn physicians. Findings of the various endpoints were:
- Sensitivity – The DeepView System demonstrated a statistically significant improvement in identifying non-healing tissue compared to burn physicians, as judged on sensitivity. At the image-wise level, the DeepView System scored 86.6% while clinical judgment annotation (CJA) of burn physicians scored 40.8%. At the pixel-wise level, the DeepView System scored 81.9% and CJA of burn physicians scored 38.8%;
- Dice Score[1] – The DeepView System achieved statistically significant higher Dice Scores when compared to those derived from burn physicians’ CJA, representing the improved pixel-wise evaluation between predicated and true segmented wound areas with the DeepView System performing at 68.5% and burn physician’s CJA at 39.2%;
- Specificity – The DeepView System produced a specificity of 61.2% (versus an anticipated result of 36.0%) and CJA of 79.1%.
The anticipated regulatory pathway will include a De Novo FDA submission for DeepView in June 2025, which, if successfully approved, will allow for commercialization of the device in 2026. Spectral will request consideration of DeepView as a Class II device.[2] After receiving the approval for use in burn centers, Spectral will pursue emergency department approval via the 510(k) pathway.[3]
Beyond the binary output provided by the DeepView Burn diagnostic, the device is also able to rapidly and accurately determine burn size. Details of a proof-of-concept module was detailed in an October 3, 2024 press release. In seconds, DeepView Burn can provide the total body surface area (TBSA) of a burn and calculate depth, area and volume assisting providers to determine the best course forward. This technology is embedded into the DeepView system and can aid clinicians in their efforts to improve patient treatment decisions. We believe that this functionality will be part of the FDA submission and formalized in the instructions for use.
2024 Financial Results Release Date Scheduled
Spectral announced its plan to report 2024 earnings results for the period ending December 31st, 2024 on Thursday, March 7th, 2025 after the market close. A conference call will be held at 5:00 pm Eastern Time to discuss results. Call in details are available in the release and investors may also access the webcast in the Events and Presentations section of the website.
Registration Statement Filed for Spectral IP IPO
Spectral AI’s wholly-owned subsidiary, Spectral IP, Inc., filed a registration statement with the SEC related to the spinoff and an initial public offering of its shares of common stock. The new company will be renamed SIM IP, Inc. The number of shares and pricing have not yet been determined. Dominari Securities is acting as the representative of the underwriters and Revere Securities is acting as the co-underwriter for the proposed offering. Further details of the offering are available in the March 20th press release and in Form S-1.
Australia Device Installations
In July 2024, Spectral announced a collaboration with PolyNovo Limited to conduct a limited deployment for burn evaluation in Australia. PolyNovo develops and sells skin substitutes and distributes them globally. The goal of the deployment is to facilitate access to Australia’s Special Access Scheme, which allows certain health practitioners to use medical devices that are not included in the Australian Register of Therapeutic Goods. Spectral is now in the process of fulfilling the requirements before the devices can be deployed down under.
The company updated the progress with this initiative in a March 6th, 2025 press release confirming that three DeepView system devices were installed in Australia. The installations will support the anticipated future rollout of additional DeepView devices based on clinician evaluations and experiences with the device.
Milestones
- Completion of burn center enrollment – August 2024
- Management restructuring – October 2024
- Warrant repricing – November 2024
- Stakeholder involvement in DeepView training & validation – December 2024
- Confirmation of compliance with NASDAQ listing requirements – December 2024
- DeepView Burn Study Topline – December 2024
- Emergency Department enrollment completed in US Burn Pivotal Study – January 2025
- Data analysis complete for US Burn Pivotal Study – February 2025
- Deployment of DeepView System in UK – 2025
- Emergency Department Enrollment Completion – April 2025
- De Novo classification request for burn diagnostic – June 2025
- Spin out and IPO of Spectral IP – 2025
- Launch of DeepView in US Burn Centers – 2026
- Launch of DeepView in US Emergency Departments (Burn) – 2026/2027
- DeepView SnapShot M Launch for Military Use – 2027
Summary
Spectral provides topline data for its burn center validation study demonstrating impressive results for its sensitivity and Dice score, which both were substantially better and statistically significant compared to assessments by burn physicians. Next steps for the program are to compile the De Novo application which should be in front of the FDA by mid-year 2025. In parallel, Spectral is developing its SnapShot M portable device and expanding its reach into Australia and the UK with new system deployments. The company is on track for a mid-2025 submission of its De Novo application, which, if accepted by the FDA, suggests approval and deployment of the DeepView burn device in 2026. We maintain our valuation of $5.00 per share.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks SCR provides and Zacks SCR receives quarterly payments totaling a maximum fee of up to $40,000 annually for these services provided to or regarding the issuer. Full Disclaimer HERE.
________________________
[1] The Dice Score, also known as the Dice Similarity Coefficient (DSC), is a metric used to measure the similarity between two sets, commonly used in image segmentation tasks to compare the predicted segmentation with the ground truth. It is commonly used in medical image segmentation, computer vision and natural language processing. The DSC contrasts with simple accuracy metrics like sensitivity and specificity in that it considers both size and spatial alignment of segmented regions, reflecting perceptual quality more effectively.
[2] Class II medical devices require greater regulatory controls than Class I devices to ensure safety and effectiveness but are typically less risky than Class III devices. The FDA classifies medical devices based on the level of control necessary to provide a reasonable assurance of the device’s safety and effectiveness. Class II medical devices present moderate risk and can pose a moderate risk to the patient if used incorrectly or malfunction. They require special controls in addition to the general controls required for Class I devices which may include specific labeling requirements, mandatory performance standards and post-market surveillance.
[3] The 510(k) pathway is designed for devices that are substantially equivalent to a legally marketed device known as a predicate device. The device must not pose new risks. 510(k) is primarily intended for Class II devices, but some low-risk Class I devices also follow this pathway. The approval process calls for the manufacturer to demonstrate that the device is similar in safety and effectiveness to an already-approved device. Clinical trials are usually not required unless substantial changes are made to the design or use.